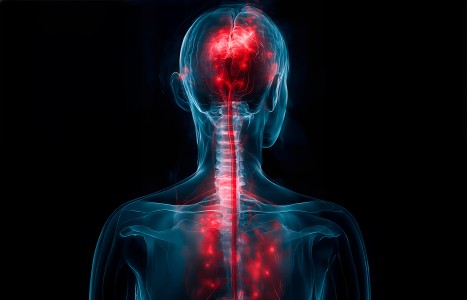
One of the longest nerves in the body is known as the vagus nerve (VN). The VN is the 10th pair of cranial nerves that originates at the brain stem in the medulla oblongata. This nerve is part of the parasympathetic nervous system, which is a part of the ANS. Research suggests ear acupuncture can activate the VN.
Supplement Associations Join Forces on Ephedra
Three of the nation's leading dietary supplement trade associations have submitted a letter to the Food and Drug Administration concerning a recent ruling by the Utah District Court on the sale of low-dose ephedra products. The letter, signed by the American Herbal Products Association, the Council for Responsible Nutrition and the National Nutritional Foods Association, was sent to acting FDA Commission Lester Crawford on May 17, 2005, and urges the FDA to address a number of "uncertainties" raised by the ruling.
On Apr. 13, 2005, the United States District Court for the District of Utah ruled in the case of Nutraceutical Corp. v. Crawford that the FDA had failed to meet the burden of proof that a dosage of 10 milligrams per day or less of ephedrine alkaloids poses an "unreasonable risk of illness of injury." The court also ruled that the FDA's use of a risk-benefit analysis to determine what constituted an unreasonable risk of supplements containing ephedrine alkaloids was "improper."
The full text of the ephedra letter can be accessed at www.ahpa.org/05_0517_Crawford.pdf.