Cartigenix:
Regenerative Pain Relief
Tom Bayne DC
Osteoarthritis (OA) is a debilitating, chronic joint disease affecting more than 32.5 million adults in the United States. As the aging population grows and obesity rates climb, this number is expected to surge. By 2030, nearly 67 million Americans will be living with arthritis, with OA being the most prevalent form1,2,4. OA is not just a condition of the joints—it’s a systemic burden that increases the risk of serious comorbidities such as cardiovascular disease, type 2 diabetes, and depression, significantly impairing quality of life and increasing healthcare costs2,3,4.
Despite the significant burden of OA, treatment options remain limited. Glucosamine is the most widely used natural remedy, with approximately 6.5 million U.S. adults taking it annually5. Yet, mounting evidence shows that glucosamine—and often paired with chondroitin—offers negligible benefit. Multiple rigorous studies and meta-analyses demonstrate no meaningful relief from pain or improved joint function compared to placebo6,7,8. A 2022 review analyzing eight high-quality studies echoed these findings, failing to identify any substantial benefit for knee OA patients9. Many of the few positive studies suffer from limitations, such as poor methodology or small sample sizes, further weakening the case for glucosamine10–13 The need for a safer, more effective solution is clear.
Randomized Controlled Trials (RCT) on a next-generation nutraceutical combining clinically standardized, novel extracts of Boswellia serrata and Apium graveolens are showing exciting changes that have never been seen before in patients with osteoarthritis.
In the first RCT, the Boswellia and celery seed combination was tested on 1,236 patients aged 19 to 75 who had not responded well to non-steroidal anti-inflammatory drugs (NSAIDs). After 90 days of supplementation, WOMAC scores—measuring pain, stiffness, and physical function—improved by 72.5%. VAS pain scores dropped by 81.4%. Even more compelling, 37% of participants experienced a 90% or greater improvement in symptoms, and 85% reported excellent to moderate overall improvement. Importantly, the treatment was well-tolerated, with no reported adverse events, marking a significant breakthrough in natural OA therapy17.
A second RCT evaluated this herbal combination in 62 adults aged 40 to 65 with clinically confirmed knee OA. Participants received either 550mg of the Boswellia and celery seed blend or placebo for 90 days. The trial assessed pain, stiffness, mobility, radiological findings, and biomarker changes. The results were striking. Recipients taking the herbal blend saw a 64% reduction in total WOMAC scores, with pain and stiffness scores dropping by 78.2% and 78%, respectively, compared to just 4.4% and 0.7% in the placebo group. VAS pain scores improved by 67.7%, declining from 6.4 to 2.1, while the placebo group saw only a 7.5% reduction.
Mobility was also dramatically improved. Participants who took the Boswellia and celery seed blend increased their six-minute walk distance by 57.46 meters, while those in the placebo group improved by just 2.13 meters. Quality-of-life measures also showed impressive gains: KOOS scores revealed significant improvements in confidence and daily function, while the FACIT-Fatigue scale demonstrated a 40.97% reduction in fatigue symptoms, signaling restored energy and vitality14.
In addition to symptomatic relief, those taking the herbal combination delivered biochemical and radiological evidence of disease modification. Cartilage degradation markers were significantly reduced—sCTX-II dropped by 41.5% and uCTX-II by 29.5%. At the same time, biomarkers for cartilage regeneration surged: PIIANP rose by 55% and PIICP by 65%. These changes indicate not just slowed degeneration but active cartilage repair14.
By contrast, glucosamine and chondroitin have consistently shown no meaningful effect on cartilage biomarkers. Multiple studies measuring before-and-after supplementation levels of CTX-II, PIIANP, or PIICP found no statistically significant changes, reinforcing the limited utility of these widely used supplements15,16. This herbal combo is the first natural product to demonstrate pain, inflammation and cartilage degeneration reduction while cartilage regeneration increased.
These biomarker shifts are critical because they signify real structural changes in the joint—not just symptom suppression. Cartilage is notoriously slow to regenerate, and interventions that meaningfully affect CTX-II, PIIANP, and PIICP represent a breakthrough in modifying disease progression rather than masking it. Patients taking the Boswellia and celery seed mix weren’t just feeling better—they were healing at a tissue level.
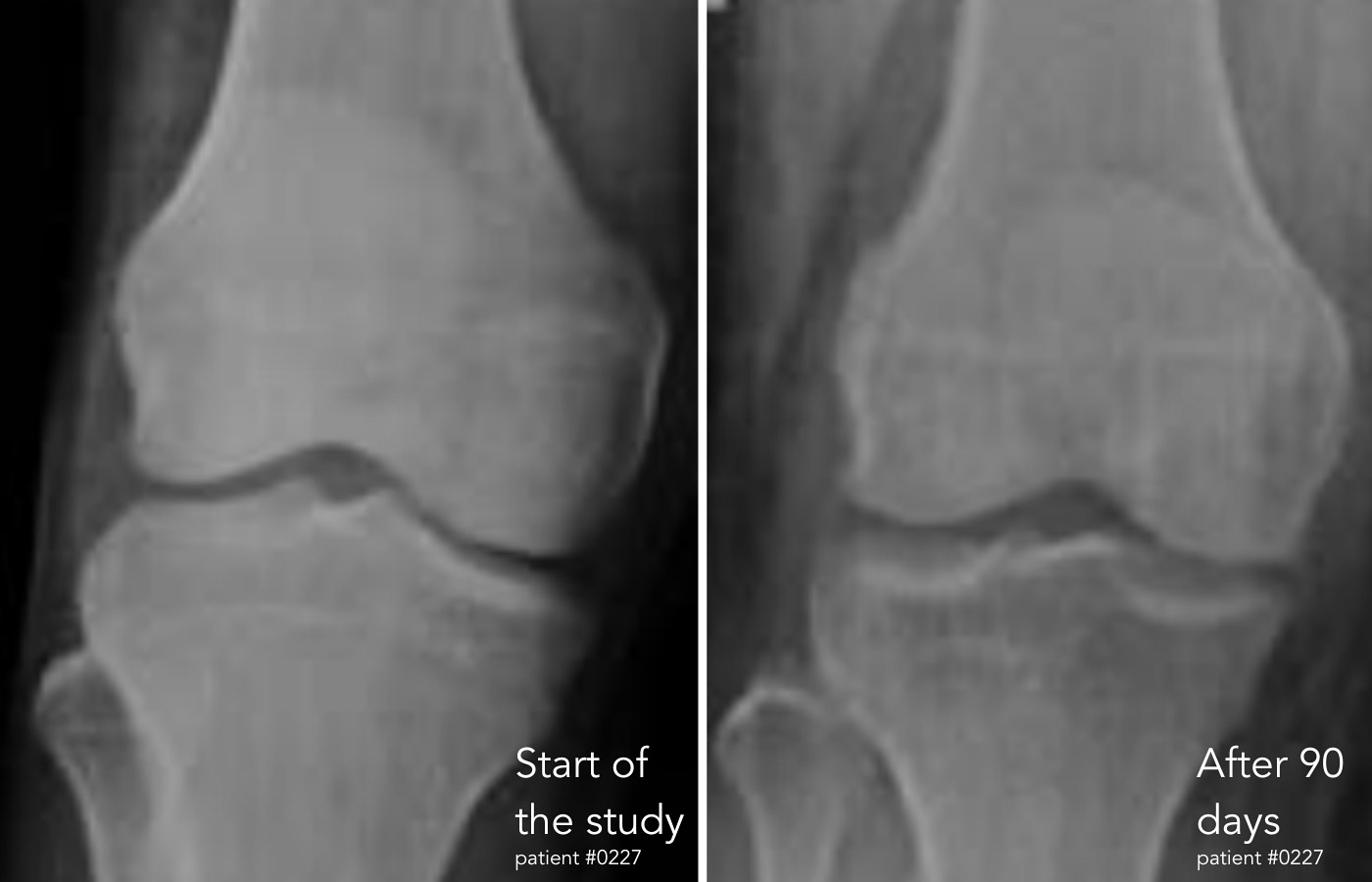
Moreover, radiological assessments supported these findings. X-rays revealed increased space between knee joints in several patients, consistent with reduced inflammation and early cartilage repair. This visual evidence aligned with both biomarker improvements and physical performance gains, forming a cohesive narrative of regeneration and recovery.
The efficacy of this herbal blend is attributed to the targeted biological activity of its components. Boswellia serrata acts by inhibiting 5-lipoxygenase and modulating key inflammatory cytokines such as TNF-α and IL-1β. Apium graveolens offers complementary mechanisms, inhibiting oxidative stress pathways and blocking NF-κB signaling. Celery seed also functions as a natural COX-2 inhibitor, reducing inflammation without the gastrointestinal side effects associated with synthetic drugs. Finally, the unique actives serratol and tirucallic acid that are standardized in this particular herbal formula, help drive tissue repair. This is powerful root cause therapy with a powerful ability to control acute symptoms.
The safety profile of this botanical combination is unmatched. Across both studies, no adverse events were reported. Unlike NSAIDs, which often cause gastrointestinal distress, the Boswellia seems to offer protective benefits. Boswellia’s active compound, serratol, has demonstrated gastroprotective properties in preclinical studies, providing an added safety advantage.
Cartigenix-HP, by Calroy, stands as a paradigm-shifting advancement in joint health management. It offers substantial, clinically proven improvements in pain, stiffness, and mobility, alongside biomarker and x-ray confirmed cartilage regeneration. With excellent safety, sustained benefits post-treatment, and powerful multi-targeted action, Cartigenix is not just an alternative—it is a superior, science-backed solution for the millions suffering from knee osteoarthritis.
The implications of these results are far-reaching. As OA continues to rise globally, driven by sedentary lifestyles, longer life expectancies, and metabolic disorders, solutions like Cartigenix could reduce the dependence on pharmaceuticals that carry long-term risks.
Patients deserve more than symptom management; they deserve restoration. Cartigenix doesn’t just delay decline—it reverses key pathological markers and improves lives. With its stellar safety profile, it’s poised to become the gold standard treatment in natural joint care.
|
At Calroy, we don’t follow trends—we set them. And we don’t just follow the science—we advance it. Calroy creates products without parallel: clinically validated, category defining solutions that support long-term health by targeting the systems and structures most others overlook. From launching the first and still gold standard endothelial glycocalyx supplement (Arterosil HP) to the first nitric oxide product to provide 24 hours of support on a single dose (Vascanox HP), we pioneer breakthroughs that deliver meaningful outcomes. Calroy is proud to bring you Cartigenix-HP, documented to regenerate cartilage in 2 clinical trials. |

Dr. Tom Bayne is a Chiropractic Physician who has practiced and worked in the field of Functional Medicine for almost 30 years. Dr. Bayne worked with a team of physicians and microbiologists to create MegaSporeBiotic®, the world's first pharmaceutical-grade, 100% spore-based probiotic. This innovation marked the beginning of Microbiome Labs, where he served as President. He traveled globally to educate healthcare practitioners on the connection between the gut microbiome and various chronic conditions.
Since his exit in 2023, Tom has Co-founded 3 other companies in the Health and Wellness space with the goal of providing cutting edge solutions to some of today’s most concerning health issues by providing clinically proven technologies and ingredients that improve human health.
REFERENCES
- Handout on Health: Osteoarthritis. National Institutes of Health. NIH publication number 06-46-17, May 2006.
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229.
- Veronese N, et al. Osteoarthritis and risk of cardiovascular disease: meta-analysis. Osteoarthritis Cartilage. 2016;24(4): 518–529.
- Behzad Heidari, Knee OA Prevalence, Risk Factors, Pathogenesis, and Features: Part 1, Caspian J of Internal Med, 2011 Spring 2(2):205-212
- Future Market Insights Report, Glucosamine Supplement Market Size, Trends, and Forecasts 2023-2033.
- Daniel Klegg MD, Crystal Harris PhD, et al, Glucosamine, Chondroitin sulfate and the two in Combination for painful knee Osteoatrthritis, The New England J Med, 2006;354::795-808
- Simon Wandel, Peter Juni, et al, Effects of Glucosamine, Chondroitin, or Placebo in Patients with Osteoarthritis of the hip and knee: network meta-analysis, BMJ 2010 Sept 16;341: c4675
- Timothy McAlindon MD, et al, Effectiveness of Glucosamine for Symptoms of knee Osteoarthritis: Results from an Internet-based randomized double blind controlled trial, The Am J of Med, Vol 117, Issue 9, 1 Nov 2004, p 643-649
- Xiaoyue Zhu, et al, Effectiveness and Safety of Glucosamine and Chondroitin for the treatment of Osteoarthritis: a meta-analysis of random controlled trials, J Orthop Surg Res. 2018 Jul 6;13:170
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol 2006;20:721-40. [DOI] [PubMed] [Google Scholar]
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med 2007;146:580-90. [DOI] [PubMed] [Google Scholar]
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum 2007;56:2267-77.[DOI] [PubMed] [Google Scholar]
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of the exclusion of patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ 2009;339:b3244. [DOI] [PMC
- Vaidya, N., Agarwal, R., Dipankar, D. G., Patkar, H., Ganu, G., Nagore, D., Godse, C., Mehta, A., Mehta, D., & Nair, S. (2025). Efficacy and safety of Boswellia serrata and Apium graveolens L. extract against knee osteoarthritis and cartilage degeneration: A randomized, double-blind, multicenter, placebo-controlled clinical trial. Pharmaceutical Research. https://doi.org/10.1007/s11095-025-03818-2
- Degradation in patients with knee osteoarthritis: Randomized discontinuation trial results employing biomarkers, J Rheumatol 2005 May; 32(5): 896-902
- Rei Momomura et al, Evaluation of the effect of glucosamine admin on biomarkers of cartilage and bone metabolism in bicycle racers, Molecular Medicine Reports, March 2013 vol 7 Issue 3.
- Dr. Anish Desai et al, Clinical effectiveness and tolerability of celery seed and Boswellia serrata extract in the treatment of knee osteoarthritis. Int J of Orthopedic Sciences, 2022; 8(2):248-252